Details of the Drug
General Information of Drug (ID: DMQXI9F)
| Drug Name |
L-Phenylalanine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Endophenyl; Fenilalanina; Phe; Phenylalanine; Phenylalaninum; Fenilalanina [Spanish]; Phenylalaninum [Latin]; Antibiotic FN 1636; CB 3208; NCI9959; Alpha-Aminohydrocinnamic acid; Beta-Phenylalanine; L-Antibiotic FN 1636; L-PHENYLALININE; N-Formylmelphalan; Phenylalanine (VAN); H-Phe-OH; L-Phenylalanine (JP15); Phenylalanine (USP/INN); Phenylalanine [USAN:INN:JAN]; Beta-Phenyl-L-alanine; N-Formyl-L-sarcolysin; N-Formyl-L-p-sarcolysin; L-PHENYL ALANINE (SEE ALSO 22839-47-0, ASPARTAME; L-3-(p-(Bis(2-chloroethyl)amino)phenyl)-N-formylalanine; L-Phenylalanine, 4-(bis(2-chloroethyl)amino)-N-formyl-(9CI); (2S)-2-amino-3-phenylpropanoic acid; (L)-Phenylalanine; (S)-(-)-Phenylalanine; (S)-2-Amino-3-phenylpropanoic acid; (S)-2-Amino-3-phenylpropionic acid; (S)-Phenylalanine; (S)-alpha-Amino-benzenepropanoic acid; (S)-alpha-Amino-beta-phenylpropionic acid; (S)-alpha-Aminohydrocinnamic acid; 1F9436B3-8B0D-4AC6-A004-4249B0BDA436; 3-Phenyl-L-alanine; 3-Phenylalanine; 3-[4-[bis(2-chloroethyl)amino]phenyl]-2-formamidopropanoic acid
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Dietary supplement
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
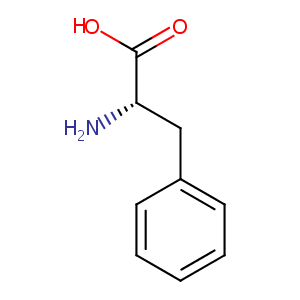 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 165.19 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| ADMET Property | |||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Malnutrition | |||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 5B50-5B71 | |||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||
References
